Researchers Cultivate Humanized Kidneys in Pig Embryos
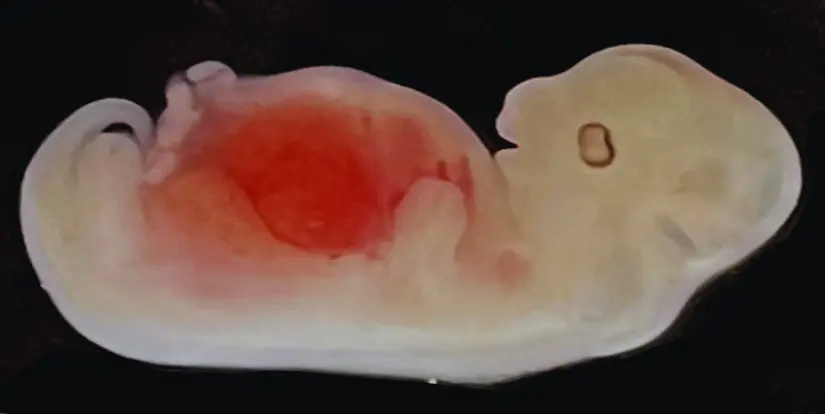
In a groundbreaking development, scientists have achieved a significant milestone in the quest to create viable human organs for transplantation.
Researchers have successfully grown kidneys primarily composed of human cells inside pig embryos. This marks the first instance of a solid humanized organ with human and animal cells cultivated within another species.
This extraordinary achievement was reported on September 7 in Cell Stem Cell, heralding a new era in human-animal chimerism.
Tao Tan, a distinguished cell biologist at Kunming University of Science and Technology in China, previously contributed to creating the first chimeric human-monkey embryo in 2021. He expressed his admiration for this remarkable feat.
The urgent need for organ transplants is a global concern, and more than 100,000 people in the United States alone are waiting for life-saving transplants. Kidney transplants are the most sought-after among these.
To address this critical shortage, scientists have been tirelessly exploring innovative methods to cultivate organs and tissues within animals.
Also Read, Ban on Disposable Vapes Proposed Amid Concerns of Child Addiction
In recent years, scientists have achieved significant breakthroughs. These include growing rat organs within mice and vice versa and cultivating humanized skeletal muscle and endothelial tissue in pigs.
However, formidable challenges persisted due to the inherent difficulty of sustaining human cells within a foreign host.
Human induced pluripotent stem cells (iPSCs), often described as a “starter kit” for growing various human tissues, frequently struggled to survive within animals due to the stark differences in physiological requirements.
The journey to this groundbreaking accomplishment began with stem cell biologist Liangxue Lai and his team at the Guangzhou Institutes of Biomedicine and Health in China.
They dedicated over five years to refining their techniques, focusing on enhancing the survivability of human stem cells within pig embryos.
In a meticulously orchestrated process, the researchers used the powerful gene-editing tool CRISPR/Cas9 to remove two genes essential for kidney development within the pig embryos.
This genetic modification created a conducive environment for human iPSCs to flourish, eventually evolving into kidney cells.
Moreover, the human stem cells were engineered to have highly active genes that inhibited apoptosis or cell death, ensuring the cells’ longevity and facilitating kidney formation.
More than 1,800 embryos were implanted into surrogate sows following these intricate preparations. Five embryos were harvested for analysis within the initial 28 days.
Astonishingly, all five embryos exhibited typically developed kidneys comprised of 50% to 60% human-derived cells.
This represents the highest percentage of human cells ever observed within a pig-grown organ. With further development, the human cells could gradually dominate the pig cells.
While this achievement is a significant and fascinating stride forward, Massimo Mangiola, a transplant immunologist at New York University Langone Health, reminds us that fully functional xenotransplants are still years away.
Differentiating stem cells into various cell types, including kidney tubular cells and developmental tissue, is a notable achievement.
Also Read, Biden Engages in Highest-Level Discussions with Chinese Leadership in Months
However, the human kidney comprises more than 70 distinct cell types, each presenting a unique challenge for scientists to replicate. Until a fully human organ is created, the risk of rejection during transplantation remains a concern.
Notably, during the experimentation process, a few iPSCs unintentionally differentiated into neural cells in the brains and spinal cords of the pig embryos.
Mangiola assures that these cells appear random and are unlikely to lead to animals with human-like brains, which would pose ethical dilemmas.
To mitigate such ethical concerns, Liangxue Lai’s team plans to modify genes responsible for steering stem cells toward neural differentiation.
Additionally, they intend to address the issue of germline cells, including eggs and sperm, which carry genetic information to offspring.
The team’s ambitious research agenda also includes the cultivation of other human organ precursors within pigs, such as the heart and pancreas.
In reflecting on their groundbreaking achievement, Lai conveys optimism about the future. “We feel that we have accomplished a milestone in the field, but this is only the first step, and many challenges remain,” he states. “We are optimistic that with time and effort, we may be able to overcome these challenges too.”
The successful creation of human-pig hybrid kidneys is a remarkable leap toward addressing the critical shortage of organs for transplantation.
While numerous challenges persist on the road to fully functional xenotransplants, this achievement offers hope for the future of organ transplantation. It also underscores the incredible potential of scientific innovation.
As researchers push the boundaries of what is possible, the prospect of saving countless lives through organ transplants grows ever closer.
Also Read, US Embassy Urges Immediate Departure of Americans from Haiti As Soon As Possible
Frequently Asked Questions – FAQs
1. What is the significance of growing human-pig hybrid kidneys?
This achievement represents a crucial step toward generating viable human organs for transplant. It also addresses the critical shortage of organs available for needy patients.
2. What challenges remain in achieving fully functional xenotransplants?
While significant progress has been made, scientists must still replicate over 70 distinct cell types found in the human kidney. This is necessary to minimize the risk of rejection during transplantation.
3. Are there ethical concerns regarding the experiment’s results?
Some iPSCs differentiated into neural cells in pig embryos. However, experts believe this is unlikely to result in animals with human-like brains, thereby mitigating ethical dilemmas.
4. What are the future goals of the research team?
The team plans to modify genes to prevent neural differentiation and address germline cells’ concerns. They also aim to grow other human organ precursors within pigs, such as the heart and pancreas.
5. How long until fully functional xenotransplants become a reality?
While this achievement is groundbreaking, it’s important to note that fully functional xenotransplants are still several years away. Researchers are diligently working to overcome the remaining challenges in organ replication and transplantation.
